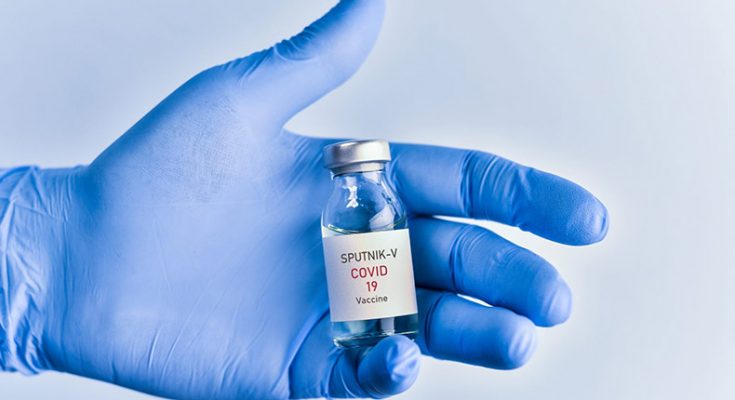
India to Approve Over 91% Effective Russian Vaccine Sputnik V Soon. Image Courtesy – Open Access Government
India is going to approve the Russian vaccine Sputnik V which is said to be over 91.6% effective based on late-stage trials in Russia. The results were published in The Lancet medical journal recently.
Indians are being vaccinated with the Covishield vaccine from the Serum Institute, a formula developed by Oxford, and the vaccine named Covaxin, a joint venture between Bharat Biotech and ICMR. Both these vaccines were given approval in the Indian market.
Dr. Reddy’s Laboratory, a Hyderabad-based vaccine manufacturer, is conducting a trial of Russia’s Sputnik V vaccine among foreign vaccines. According to the company, the results of this vaccine trial are quite good. The Sputnik V vaccine will be available in the country from March to April, and the vaccine will be sold to the government at a lower price.
Dr. Deepak Sapra, Senior Vice President of Dr. Reddy’s Laboratories Ltd., said that mass vaccinations would begin as soon as the people of the country were sure that the Sputnik vaccine was safe and secure. Reports of each stage of the trial will be submitted to the Central Drug Regulatory Authority. Dr. Sapra told that there have been no problems with the vaccine trial so far.
The Sputnik vaccine was developed by the Gamaleya Research Institute of Epidemiology and Microbiology of Russia. An agreement has been signed with Dr. Reddy’s Laboratory for the production and distribution of this vaccine in India under the supervision of the Russian Direct Investment Fund (RDIF). Gamaleya has signed a contract for a dose of around Rs 200 million in the first phase. A joint statement from RDIF and Dr. Reddy’s said that safety and controlled trials of the vaccine were being conducted in India.
Read: COVID-19 infection may happen before two doses of Pfizer’s vaccine
A small number of volunteers were monitored with a dose of the Sputnik V vaccine. G V Prasad, Co-Chairman and Managing Director of Dr. Reddy’s Lab, told that safety has been verified before applying the Sputnik V vaccine. Safety trial reports will be submitted in each phase.
Russia’s Gamaleya Research Institute of Epidemiology and Microbiology claims that the Sputnik V vaccine has been shown to be effective in 91.6% percent of cases, after the US pharmaceutical company Pfizer announced that their vaccine was 90 percent effective. The Russian company has claimed that the dose of this vaccine is increasing the immunity level of the volunteers.
The Lancet, one of the world’s most popular medical journals, reports on the effectiveness of the Sputnik vaccine. According to the study, six people were given two doses of the vaccine. The experimental application of the vaccine lasted for 42 days. The process of antibody production begins within 21 days of the first dose. The researchers claim that the second report was available 28 days after the trial. It has been observed that T-cells or T-lymphocyte cells in the blood become active immediately after the injection of the vaccine. The Lancet reports that the vaccine is 91.6% effective.
More than 1 million people in Russia already received the Sputnik V vaccine. The vaccine has been marketed by the Russian Direct Investment Fund (RDIF) in foreign countries. The vaccine has been approved in Argentina, Mexico, Algeria, Serbia, and Bolivia for emergency use.